Illustration by Steven Noble

Illustration by Steven Noble
You measure time. You are both the keeper and record, the extraordinary consequence of synchronies in series, a symphony of coordinated rhythms and loops: proteins polymerize, ions flow, spindles align, cells mitose, hormones surge, vesicles fuse, alveoli inflate, heart beats, neurons fire, gait steps, fingers tap, wounds heal, hair grays, telomeres shorten, perceptions prime, behaviors habituate, and memories form and fade. The pace of life means you rarely attend to its cycles. Periodically your origins interrupt; you are humbled by jet lag, stubble, menstruation, death. As an amalgamation of chemical reactions, you should not be happening. Every drawn breath is a laugh in the face of time, endowed by time’s lifelong trickster: the enzyme. A few degrees too many, they denature and you stop. Time is the reason you sweat.
As time-keepers, as with most things, we are most reliable unconscious. Even in the absence of external cues, the zeitgebers, our internal rhythm, oscillates around twenty-four hours and eleven minutes. The orchestration or precision timing required to traverse traffic, shake a hand, or utter a word lies below our threshold of awareness, in the order of milliseconds. And milliseconds matter. In conditions such as Parkinson’s disease, schizophrenia, and autism, where time-perception is perturbed, to move is to stammer. Stuttering eyeblinks syncopate facial and verbal micro-expressions, opaquing subtle social cues. Life is rendered a series of missed beats.
For the arachnophobe, eternity is a spider. Because our conscious perception of time is a construct that relies on memory, it is, like memory, malleable—vulnerable to distortion by emotion, attention, pharmacology, temperature, and sweat. Stimulants speed it up. Depressants and anesthetics slow it down, as do fear, sadness, and pain. Though our happiest instants may be experienced as relatively fleeting, we attribute, in retrospect, as if by some sanity-saving mechanism, far more time to them than perceived eons, lingering longer on vacations, love affairs, and first experiences than traffic jams, bladder states, or bad jokes. The valence of tedium rarely smarts. Amongst a lifetime of tears, the boredom-induced rank low.
Models for the subjective perception of time, or interval timing, propose an internal pacemaker that produces time units which accumulate, ultimately to be compared to stored reference time durations. What and where is this pacemaker? These reference times? Is it the basal ganglia spiny neurons, with their repetitive firing rate? Maybe. Or the anterior insular cortex, which seems to integrate information of all things self? Lesion and imaging studies present many possible candidates for the location of pacemakers and accumulators. The finding, or its lack, of a single time-sense organ suggests that time is perceived over distributed regions, perhaps in a number of ways.
What time should I set the alarm? Subway or cab? Can I see myself living in this apartment or city? Prospection, or future-thinking, includes simulation, prediction, intention, and planning. Reliant on memory, prospection is also shaded by emotion. Depressed people have goals and place importance on them, but they are less positive about their occurrence, feel they have less control over them, and forebode negative events as more likely to happen. Life appears hopeless. The nondepressed are overoptimistic, consistently underestimating the time required to complete tax forms, assemble furniture, and build buildings. Next time you are late, blame it on the planning fallacy. Affective forecasting is our ability to anticipate the emotional impact of a future event. We are good at predicting quality (positive or negative), but not quantity (how positive or negative), underestimating the benefits of positive interactions with strangers and the pain of being socially excluded or mildly insulted.
Can goldfish be impulsive? Bacteria patient? Do adolescent lobsters molt recklessly? Do self-sabotaging starlings procrastinate their migration? Because we can conceive of a future, we can plan for and anticipate one, foregoing immediate rewards for ultimate payoffs. Delayed gratification is context dependent and follows a delay discounting rule: the larger the reward, the longer we can wait. Imagining a positive future self is more difficult for the depressed and stressed. Patience and tenacity are blunted. Projects and diets are readily abandoned. The focal length of an addict’s horizon is limited to the next hit.
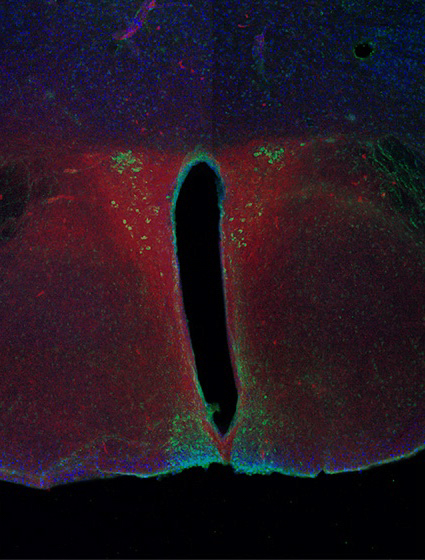
Stained neurons in the region of the brain that controls circadian rhythm; image provided by the author
Time is tied to metaphor, bound in space. We move through time. It flows past us. The sun rises in the east, the moon waxes and wanes, but the way we know night follows day, death follows birth, and more crucially, lunch follows breakfast—he formation of our spatiotemporal maps—is less influenced by the geometry of the heavens and more by the language we speak. Time cells and place cells are neighbors in the brain, both residing in the hippocampus, the brain structure essential for memory. Charting time with space is universal across languages, but the direction we write dictates our mental timeline: English is left to right, and Mandarin can be both horizontal and vertical. For larger epochs, all cultures think dorsal-sagittally, looking ahead to the future with the past behind them. All cultures apart from one: Aymara speakers see the future as behind them, because the future is unknown. Elderly Aymara people refuse to talk about the future on the grounds that little or nothing sensible can be said about it. They’re right. The future is imagined, hypothetical. But hypotheticals exist in all tenses. Our mind is a constellation of conditionals and counterfactuals. If time is a line, then truth is a vector.
Imagining the future is remembering the past. The processes are symmetrical, engage the same brain structures, and develop in parallel. An amnesiac fails at both. We deconstruct our semantic memories (what we know about the world) and our episodic memories (what has happened to us) and recombine them with intention. We remember what hasn’t happened. To conceive of who we were, are, and will be is essential to the construction and continuation of the self. We can mentally time travel abstractly—chronosthesia—to think of a past and a future, but it is our autonoetic awareness, our synthesis of personal memory and intention, that enables us to not only conceive of the future but to project ourselves into it, with feeling. To maintain selfhood, we overlap considerably with our near-future iterations, but the further we project in time, the more tenuous this imaginary string of selves becomes, until it threatens to be severed, such that we appear other. Order follows time’s arrow into chaos. But humans love to order. As molecules sequentially arrange themselves into structures of increasing complexity, we temporally arrange perceptions and movements into gesture, language into narrative. Life defies decay. Narrative defies forgetting. Both continue to defy erasure.
Leah Kelly joined artist Shezad Dawood to further explore nonlinear narratives and the negative and positive influences of nostalgia in the program The Science of Nostalgia at the Rubin Museum on February 24, 2018.
Body Clock
Czeisler, C. A., J. F. Duffy, T. L. Shanahan, et al. “Stability, Precision, and Near-24-Hour Period of the Human Circadian Pacemaker,” Science 284, no. 5342 (1999): 2177–81.
Thoenes, S., and D. Oberfeld. “Meta-Analysis of Time Perception and Temporal Processing in Schizophrenia: Differential Effects on Precision and Accuracy.” Clinical Psychology Review 54 (2017):4464.
Allman, M. J. “Deficits in Temporal Processing Associated with Autistic Disorder.” Frontiers in Integrative Neuroscience 5 (2011): 2.
Avanzino, L., E. Pelosin, C. M. Vicario, G. Lagravinese, G. Abbruzzese, and D. Martino. “Time Processing and Motor Control in Movement Disorders.” Frontiers in Human Neuroscience 10 (2016): 631.
Meck, W. H. “Neuropharmacology of Timing and Time Perception.” Cognitive Brain Research 3 (1996): 227–42.
Buhusi, C. V., and W. H. Meck, “Differential Effects of Methamphetamine and Haloperidol on the Control of an Internal Clock.” Behavioral Neuroscience 116 (2002): 291–97.
Burle, B., and L. Casini. “Dissociation between Activation and Attention Effects in Time Estimation: Implications for Internal Clock Models.” Journal of Experimental Psychology: Human Perception and Performance 27, no. 1 (2001): 195–205.
Bar-Haim, Y., A. Kerem, D. Lamy, and D. Zakay. “When Time Slows Down: The Influence of Threat on Time Perception in Anxiety.” Cognition and Emotion 24 (2010): 255–63.
Lake, J. I. “Recent Advances in Understanding Emotion-Driven Temporal Distortions.” Current Opinion in Behavioral Sciences, 8 (2016): 214–19.
Aschoff, J. “Human Perception of Short and Long Time Intervals: Its Correlation with Body Temperature and the Duration of Wake Time.” Journal of Biological Rhythms 13 (1998): 437–42.
Brown, S.W. “Attentional Resources in Timing: Interference Effects in Concurrent Temporal and Nontemporal Working Memory Tasks.” Perception and Psychophysics 59 (1997): 1118–40.
Droit-Volet, S., and W. H. Meck. “How Emotions Colour Our Perception of Time.” Trends in Cognitive Sciences 11 (2007): 504–13.
Droit-Volet, S., S. Brunot, and P. M. Niedenthal. “Perception of the Duration of Emotional Events.” Cognition and Emotion 18 (2004): 849–58.
Gibbon, J., R. M. Church, and W. H. Meck. “Scalar Timing in Memory.” In Timing and Time Perception, edited by J. Gibbon and L.G. Allan, 52–77. New York: New York Academy of Sciences, 1984.
Treisman, M., A. Faulkner, P. L. Naish, and D. Brogan. “The Internal Clock: Evidence for a Temporal Oscillator Underlying Time Perception with Some Estimates of its Characteristic Frequency.” Perception 19, no. 6 (1990): 705–43.
Matell, M. S., and W. H. Meck. “Cortico-Striatal Circuits and Interval Timing: Coincidence Detection of Oscillatory Processes.” Cognitive Brain Research 21 (2004): 139–70.
Craig, A. D. “Emotional Moments Across Time: A Possible Neural Basis for Time Perception in the Anterior Insula.” Philosophical Transactions of the Royal Society B: Biological Sciences 264 (2009): 1933–42.
The Prepared Mind
Schacter, D. L., R. G. Benoit, and K. K. Szpunar. “Episodic Future Thinking: Mechanisms and Functions.” Current Opinion in Behavioral Sciences 17 (2017): 41–50.
Szpunar, K. K., R. N. Spreng, and D. L. Schacter. “A Taxonomy of Prospection: Introducing an Organizational Framework of Future-Oriented Cognition.” Proceedings of the National Academy of Sciences USA 111 (2014): 18414–21.
Miloyan, B., and T. Suddendorf. “Feelings of the Future.” Trends in Cognitive Sciences 19, no. 4 (2015): 196–200.
Rish, M., O. Piguet. “The Pivotal Role of Semantic Memory in Remembering the Past and Imagining the Future.” Frontiers in Behavioral Neuroscience 7 (2013): 27.
Buehler, R., D. Griffin, and M. Ross. “Exploring the “˜Planning Fallacy’: Why People Underestimate Their Task Completion Times.” Journal of Personality and Social Psychology 67 (1994): 366–81.
Anderson, R. J., and G. L. Evans. “Mental Time Travel in Dysphoria: Differences in the Content and Subjective Experience of Past and Future Episodes.” Consciousness and Cognition 37 (2015): 237–48.
Dickson, J. M., N. J. Moberly, and P. Kindermann. “Depressed People Are Not Less Motivated by Personal Goals but Are More Pessimistic about Attaining Them.” Journal of Abnormal Psychology 120(2011): 975–80.
Berns, G. S., D. Laibson, and G. Loewenstein. “Intertemporal Choice—Toward an Integrative Framework.” Trends in Cognitive Sciences 11 (2007): 482–88.
Peters, J., and C. Büchel. “Episodic Future Thinking Reduces Reward Delay Discounting through an Enhancement of Prefrontal-Medio Temporal Interactions.” Neuron 66, no. 1 (2010): 138–48.
The Natural Order
Bonato, M., M. Zorzi, and C. Umiltà. “When Time Is Space: Evidence for a Mental Timeline.” Neuroscience and Biobehavioral Reviews 36 (2012): 2257–73.
Lai, V. T., and L. Boroditsky. “The Immediate and Chronic Influence of Spatio-Temporal Metaphors on the Mental Representations of Time in English, Mandarin, and Mandarin-English Speakers.” Frontiers in Psychology 4 (2013): 142.
Núñez, R. E., and E. Sweetser. “With the Future Behind Them: Convergent Evidence from Aymara Language and Gesture in the Cross Linguistic Comparison of Spatial Construals of Time.” Cognitive Science 30 (2006): 401–50.
De Brigard, F., D. R. Addis, J. H. Ford, D. L. Schacter, and K. S. Giovanello. “Remembering What Could Have Happened: Neural Correlates of Episodic Counterfactual Thinking.” Neuropsychologia 51, no. 12 (2013): 2401–14.
Block R. A., and D. Zakay. “Prospective and Retrospective Duration Judgments: A Meta-Analytic Review.” Psychonomic Bulletin Review 4 (1997): 184–97.
Gilead, M., N. Liberman, and A. Maril. “Construing Counterreal Worlds: The Role of Abstraction.” European Journal of Social Psychology 42 (2012): 391–97.
Rathbone C. J., M. A. Conway, and C. J. A. Moulin. “Remembering and Imagining: The Role of the Self.” Consciousness and Cognition 20, no. 4 (2011): 1175–82.

Leah Kelly is a neuroscientist at Rockefeller University. She uses electrophysiology, tracing, and imaging to elucidate how neurons communicate with the rest of the body to regulate appetite and metabolism. In 2014 she cocurated the Impakt Festival in the Netherlands. Her essay “Sense of Self” appears in Experience: Culture, Cognition, and the Common Sense, published by MIT in 2016.
Get the latest news and stories from the Rubin, plus occasional information on how to support our work.